As legalization spreads, researchers scramble to understand long-term impacts.
In late 2020, a group of researchers in the UCSF Department of Anesthesia and Perioperative Care received a $2 million grant from the California Bureau of Cannabis Control (BCC) to look at the developmental effects of cannabis exposure on the immune, central nervous and peripheral nervous systems. The grant was the largest of $30 million in public university research grants the BCC issued to universities across California.
Titled Comprehensive Analysis of Developmental Cannabis Exposure on Brain, Immune, and Sensory Systems, principal investigators from the department’s faculty and staff include Roland Bainton, MD, PhD; Judith Hellman, MD; lead investigator Jeffrey Sall, MD, PhD; Jennifer Sasaki Russell, PhD, and Mark Schumacher, MD, PhD.
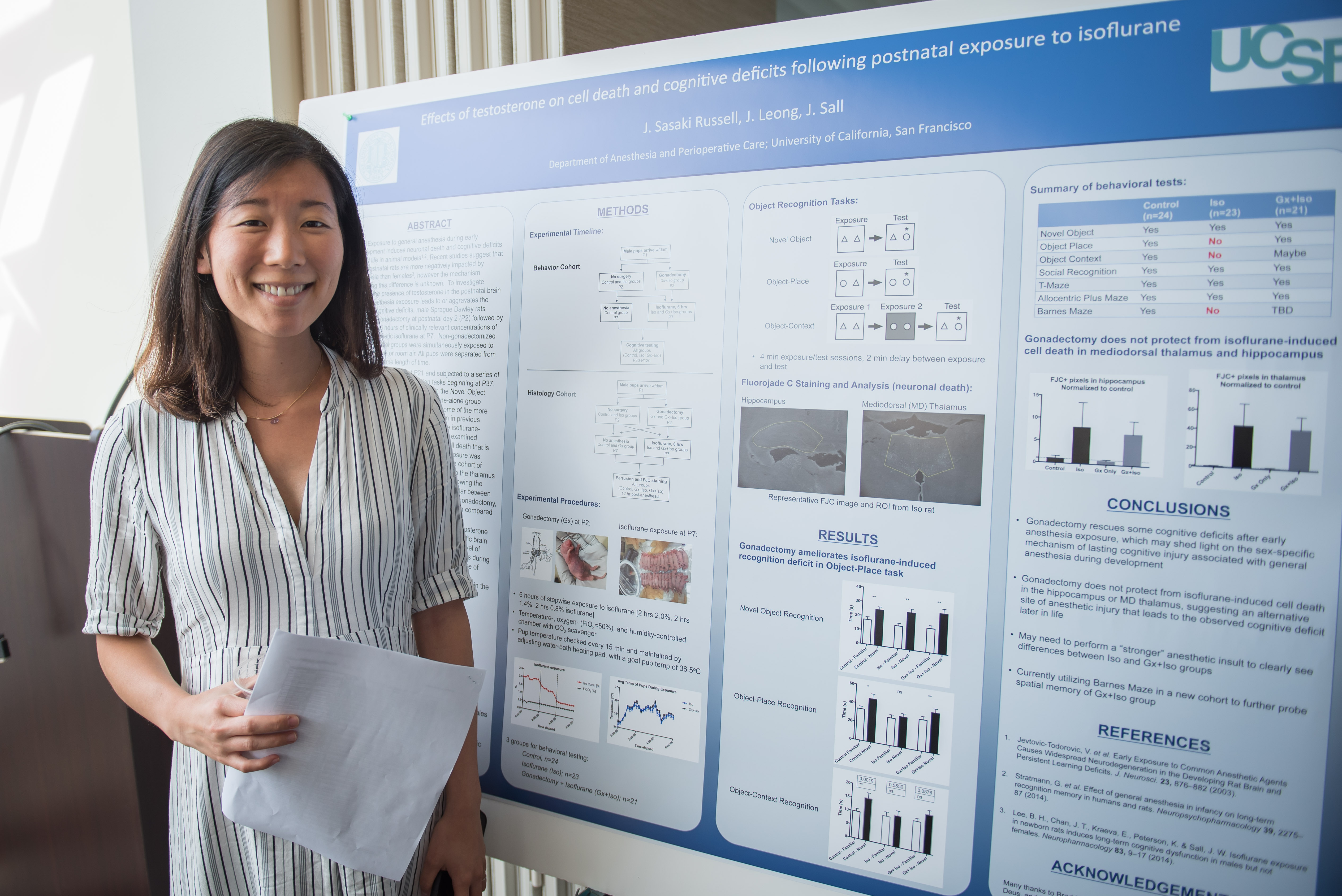 Sasaki Russell (pictured, left), a senior researcher in the Sall Lab, says the work is important because there is little known about the long-term effects of cannabis on human development, despite increasingly widespread legalization. The endocannabinoid system – a series of receptors and lipids either activated or deactivated by the presence of THC and lesser-known cannabinoids – was only discovered in the late 1990s, so cannabinoids’ role in modulating acute and chronic processes in health and disease is really in its infancy. And until recently, research using the formerly illegal Schedule 1 drug faced a number of barriers.
Sasaki Russell (pictured, left), a senior researcher in the Sall Lab, says the work is important because there is little known about the long-term effects of cannabis on human development, despite increasingly widespread legalization. The endocannabinoid system – a series of receptors and lipids either activated or deactivated by the presence of THC and lesser-known cannabinoids – was only discovered in the late 1990s, so cannabinoids’ role in modulating acute and chronic processes in health and disease is really in its infancy. And until recently, research using the formerly illegal Schedule 1 drug faced a number of barriers.
“Yet because it’s been around so long, many people don't believe cannabis could be harmful enough to explore in this way; others don’t believe it could have genuine medical utility,” says Hellman. “We believe we need to know how any drug in use affects different systems.”
Schumacher agrees. He says part of the original push for marijuana’s therapeutic use came from anecdotes and research indicating the drug offered pain and other symptom relief, especially for patients undergoing cancer treatment. As the department’s chief of the division of Pain Medicine, Schumacher is intensely interested in developing alternatives to opioid treatments but, he says, “We don’t have a good sense of why cannabinoids work for some and not others, much less their effect on early childhood or development, or the way its therapeutic effect in one system could have side effects in others. This is concerning and just drives home that we don’t know what the long-term effects of this drug are.”
With legalization now a reality in many places, the team hopes that research can help sync behaviors and regulations with scientific knowledge of the drug’s effects, in the same way studies on alcohol and tobacco changed how many people use those products.
“We are hoping to generate questions and hypotheses for further study of the developmental effects of cannabis,” says Sall. “But we also hope that we can begin to answer some existing questions by standardizing some dosing and delivery across different experiments and observing the effects across the three systems.”
Approaching Questions from Multiple Angles
 Schumacher’s (pictured, left, facing camera) expertise is on the peripheral nervous system, Hellman’s the immune system, and Sall’s the developmental effects of drugs on the central nervous system. Bainton will oversee genetic sequencing for all of the studies to understand how early or later exposures might change gene expression.
Schumacher’s (pictured, left, facing camera) expertise is on the peripheral nervous system, Hellman’s the immune system, and Sall’s the developmental effects of drugs on the central nervous system. Bainton will oversee genetic sequencing for all of the studies to understand how early or later exposures might change gene expression.
While some of the researchers are relatively new to studying cannabis, Hellman has been studying the endocannabinoid system for almost a decade.
“Cannabis, of course, is known for its psychotropic, behavioral, and neurologic effects, and, more recently, for controlling chronic inflammatory conditions. My group’s research originally focused on the immune effects of cannabis-derived cannabinoids in models of acute inflammation,” says Hellman. “We then began to explore how cannabinoids regulate inflammation. What makes different cannabinoids produce more or less inflammation and how do they affect baseline immune function and response? These are all wide-open areas of investigation.”
Her prior work with THC revealed profound anti-inflammatory effects that, says Hellman, can be a double-edged sword. On the one hand, suppressing inflammation can be protective, but inflammation is also necessary for a proper immune response. “We need the right kind of functional inflammation and it’s hard to say yet whether the anti-inflammatory effects we see are a good thing.”
 On this project, Hellman (pictured, left) will zero in on the effects of minor cannabinoids and terpenes, particularly CBD and CBG, to understand how exposures at different times in the lifespan affect infection and inflammation. She will use cells derived from autopsy samples or healthy human volunteers across the lifespan. She will also conduct experiments aimed at better understanding an established connection between the nervous and immune systems.
On this project, Hellman (pictured, left) will zero in on the effects of minor cannabinoids and terpenes, particularly CBD and CBG, to understand how exposures at different times in the lifespan affect infection and inflammation. She will use cells derived from autopsy samples or healthy human volunteers across the lifespan. She will also conduct experiments aimed at better understanding an established connection between the nervous and immune systems.
That’s where Schumacher’s work on understanding how the cannabinoid receptors work on primary sensory neurons, which are dedicated to detecting painful stimuli, and their connection to inflammation come into play.
“On the clinical side, we continue to struggle with how best to manage pain and we are constantly searching for more accessible options,” says Schumacher. “In many cases, folks that have tried and failed standard therapies, including opioids, or who have had substance use disorders, have turned to cannabis products, especially CBD. But if, for example, pregnant women are consuming these agents, they may not be aware that they could actually change the nervous system for pain sensing in the fetus resulting in long -term consequences into adulthood, ”
There’s a similar concern about young people smoking cannabis or consuming edibles: in adulthood, these young people could interpret or detect even modest stimuli as being much more painful than they might have otherwise. Thus Schumacher’s work on this grant will look closely at whether early exposure to cannabis changes how we detect pain. It’s a logical starting point, since scientists do know that in utero and early infancy, sensory neurons are still dividing, creating much more neuroplasticity.
“We know, for example, that newborns who undergo repeated blood draws, experience long-lasting changes in their sensory systems, including more pain sensation. It may be that…in adulthood, it turns out to be less of an issue, but we just don’t know at this point. We do observe, however, that in the hospital, certain adult patients who are high-volume users of high-potency THC products have a much more difficult time with post-operative pain.”
Systems Approach Promises Numerous Advantages
The team is especially hopeful that this collaborative, multi-system approach will add up to more than the sum of the individual expertise of each of the investigators.
“That’s the real strength of this proposal, the hope that new insights can emerge from breaking out of traditional silos,” says Sasaki Russell. “Increasingly, the NIH (and other funders) likes to see qualified scientists working together on problems like these.”
The interaction among the systems may be particularly important. If you are taking cannabis to improve pain control, but it affects your ability to memorize or function or increases the risk of infection or pain intensity, you want to know that before using the drug.
“We are hoping that maybe there is a sweet spot – an intersection of timing and type and potency - that minimizes the effects on long-term changes in pain perception and the immune and cognitive systems so that therapeutic use becomes a realistic alternative for some conditions,” says Schumacher. “But unless we see the whole picture, we might be misleading folks.”
“The multi-system approach is also a really nice way for trainees in the department to get multidisciplinary exposure,” says Hellman, the department’s vice chair of research.
 “We are very excited,” says Sall (pictured, left). “We have several people with labs housed closely together and we’ve wanted to do this type of thing for a long time because it makes so much sense scientifically, practically, and logistically. It dramatically reduces the resources required for this research and really enhances the value of the work we are all doing.”
“We are very excited,” says Sall (pictured, left). “We have several people with labs housed closely together and we’ve wanted to do this type of thing for a long time because it makes so much sense scientifically, practically, and logistically. It dramatically reduces the resources required for this research and really enhances the value of the work we are all doing.”
