Liver Transplant Goals and Objectives for Anesthesia Residents
V 5.0
September 2022
Goals and Objectives for Anesthesia Residents on the Liver Transplant Service
Introduction
Anesthesia residents at UCSF perform anesthesia on the liver transplant service as part of the senior resident rotation and during night call as the senior resident. The liver transplant service is a multidisciplinary service comprised of surgeons, hepatologists, anesthesiologists and nurses. Approximately 150 liver transplants are performed each year, providing each resident with roughly 8 transplants each.
Goals
- Describe the surgical course of a liver transplant.
- Understand the management of a donor hepatectomy.
- Understand the preoperative evaluation of a patient with cirrhosis.
- Understand blood component therapy.
Objectives
Interpersonal Communication Skills
- Communicate effectively with other healthcare professionals.
- Demonstrate professionalism and interpersonal/communication skills with patients, families, and children.
- Communicate with patients and their families in easily understood and culture-sensitive language.
- Work effectively as a member of the liver transplant team.
- Maintain comprehensive, timely, and legible medical records.
Professionalism
- Demonstrate respect, compassion and integrity.
- Demonstrate a commitment to excellence and on-going professional development.
- Demonstrate a commitment to ethical principles pertaining to confidentiality of patient information, informed consent, and resource utilization.
- Demonstrate sensitivity and responsiveness to patients’ culture, age, gender, and disabilities.
- Demonstrate organizational skills to care for patients in a competent and efficient manner.
Liver Specific Medical Knowledge
- List what vessels are “cross” clamped during the anhepatic stage.
- Define venovenous bypass.
- Define a “piggy back” transplant.
- Define the “anhepatic period”.
- Distinguish heterotopic transplant from orthotopic transplant.
Cirrhosis
- List the factors that are used to calculate the MELD and MELD-Na score.
- List several factors used to determine Childs-Pugh classification.
- Define portopulmonary hypertension.
- Define hepatopulmonary syndrome.
- Define the approximate perioperative mortality in a patient with cirrhosis.
- List elements of coagulation abnormalities in a patient with cirrhosis.
Transfusion
- Define indications for fibrinogen concentrate.
- Define indications for prothrombin complex concentrate (PCC).
- Define indications for Factor VIIa use.
- Define the indication for use of FFP.
- Define the indication for use of cryoprecipitate.
- Define the indication for use of platelets.
- Define “massive transfusion.”
- Define dilutional coagulopathy and the associated hemostatic abnormalities.
- List blood components most associated with citrate toxicity.
Practice-based Learning and Improvement
- Perform literature search and retrieve relevant literature.
- Access UNOS website and relevant data.
Systems-based Practice
- Demonstrate awareness of the role transplantation plays in the health care system.
- Understand efforts being made to expand organ donation, both living and deceased.
Preoperative Evaluation
The severity of illness and prognosis of patients with chronic liver disease can be estimated by a number of different scoring models including the Childs–Pugh–Turcotte score, MELD score, and MELD-Na score. The latter is now widely used in the United States for the allocation of organs. It is based on a predicted 3-month mortality for patients awaiting a liver transplant, and uses four laboratory values to generate a score, which determines priority. The four laboratory values used are serum bilirubin, serum creatinine, INR, and serum sodium. The format for the three systems are as follows:
|
Variable |
1 Point |
2 Points | 3 Points |
|
Bilirubin (mg/dL) |
<2 |
2-3 |
>3 |
|
Albumin (g/dL) |
>3.5 |
2.8-3.5 |
<2.8 |
|
Encephalopathy grade |
None |
1-2 |
2-4 |
|
Ascites |
Absent |
Slight |
Moderate |
|
PT (sec prolonged) |
<4 |
4-6 |
>6 |
Child-Pugh Score: Class
A: 5-6 Class B: 7-9
Class C: 10-15
MELD (MELD Calculator): 0.957 • Ln (Creatinine, mg/dl) + 0.378 • Ln (Bilirubin, mg/dl) + 1.120 • Ln (INR) + 0.643 •Ln (cause of cirrhosis)
MELD-NA: MELD + 1.32 x (137 – serum sodium (mmol/l)) – [0.033 x MELD x (137 – serum sodium (mmol/l))]
UNOS has made the following modifications to the score:
If the patient has been dialyzed twice within the last 7 days, then the value for serum creatinine used should be 4.0.
Patients with a diagnosis of liver cancer will be assigned a MELD score based on the median MELD at transplant at the listing transplant center.
Organ System-Specific Considerations
Neuro
Hepatic encephalopathy should be assessed. In acute liver failure patients, you should look for signs of high ICP. Find out if the patient has had a CT scan looking for cerebral edema. Rarely some patients may have ICP monitoring. Often, these patients are intubated for coma.
Pulmonary
Gas exchange problems are common. While hepatopulmonary syndrome (intrapulmonary shunting from liver failure) may occur, most causes of gas exchange deficiencies are due to standard causes. These may include atelectasis, decrease FRC from ascites, pneumonia, underlying lung disease, and pleural effusions (hepatic hydrothorax) that are common in these patients. Often the effusions can be drained through the diaphragm after incision, so despite a bad effusion, the problem will not ultimately be severe during surgery.
Pulmonary Hypertension (Portopulmonary syndrome)
This is listed between the pulmonary and cardiac system, because it is a consideration for both organs, and is critically important. Cirrhosis is associated with pulmonary hypertension similar to primary pulmonary hypertension. The cause is not clear. However, these patients may do poorly, and severe enough forms may be a contraindication to transplantation. Pulmonary hypertension is difficult to screen for preoperatively, and can be asymptomatic. All patients will have had a cardiac echo, which should have estimated PA pressures. Since echo may be a poor screening tool, right heart catheterizations may be performed preoperatively and/or PA catheters may be placed to specifically look for pulmonary hypertension in high risk patients. We are currently placing PA catheters only when clinically indicated.
Cardiac
Most patients have had some cardiac evaluation. Liver transplantation is extremely stressful surgery, and will not be tolerated by a patient with significant coronary artery disease. There is a low threshold for obtaining left and right cardiac caths. MI’s have occurred in these patients. Cardiac rhythm problems are also common. Cardiac work-ups from patients referred from outside are usually found under “Care Everywhere” or “Scanned Outside Documents” in Apex or may need to be requested from the liver transplant service.
Evaluation of cardiac function is also essential. In the vasodilated state of liver failure, cardiac function should be hyperdynamic, with an elevated ejection fraction, often 70-80%. Even a mildly reduced ejection fraction, e.g. 55%, may be distinctly pathologic.
Another disease associated with liver failure with important cardiac considerations is hemochromatosis. Iron infiltration of the heart can impair cardiac contractility and conduction. Hemochromatosis is common, and because of additional diseases such as hepatitis C, it may be missed. In any adult patient with IDDM that developed after cirrhosis, hemochromatosis (bronze diabetes) should be suspected.
GI
History of GI bleeding, ascites, portal hypertension, TIPS performed should be noted. While NPO status is certainly ascertained, we consider that most patients may have full stomachs due to poor emptying with ascites, and possible GI bleeding. A Minnesota tube may be necessary during surgery should large volume variceal bleeding occur.
Renal
Hepatorenal syndrome and other causes of renal insufficiency should always be assessed. Some patients may be scheduled for combined liver and kidney transplantation. Because large volumes of FFP may be necessary, some form of fluid removal (diuresis, or CRRT) may be necessary.
Every electrolyte may be abnormal and should be considered. Hyperkalemia and hypokalemia are both common due to renal failure and diuretic use. Hyponatremia is common due to diuretic use, low intravascular volume and excessive free water retention and/or replacement. Although debated, central pontine myelinolysis may be related to over-rapid correction of hyponatremia and has been reported in the setting of liver transplantation. Mg+2 and ionized Ca+2 levels should be noted and corrected prior to the anhepatic phase.
Heme
Obvious focus on coagulation factors, hemoglobin level and platelet count are important. This should be anticipated during the preop evaluation. We currently order 5 RBC, 5 FFP and 2 plt in room before start of the lower risk cases and 10 RBC, 10 FFP and 2 plt for higher risk cases. More products can be ordered in patients who are severely coagulopathic pre-operatively and/or represent a substantial risk of bleeding from a surgical standpoint (redo liver transplants). Discuss with your attending.
The Operation: General Considerations
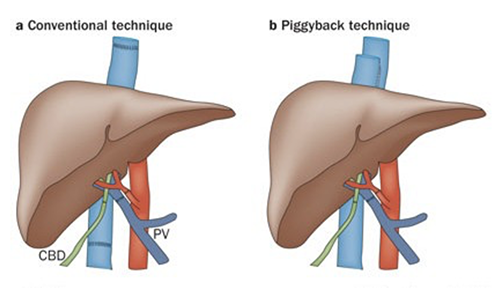
Liver transplantation is almost always performed as an "orthotopic liver transplantation", meaning the transplanted liver is placed in the same position as the native liver (compare with renal transplantation where the kidney is transplanted into a heterotopic location, the pelvis). Auxiliary liver transplantation is a technique that may be used in the future in the setting of fulminant liver failure. In our pediatric patients, our surgeons use a variety of "reduced-size"grafts: pare-down segments from adult donors, small segments (left lateral segment) from live donors, or "split-livers" -two grafts from one donor. Adult-to- adult living related transplants use donor right or left hepatic lobes. A "piggy- -back" refers to partial occlusion of the IVC intraoperatively, with an end-to-side anastomosis, instead of insertion of donor IVC. However, regardless of the surgical technique, liver transplantation involves an abdominal dissection, removal of the native liver, placement of the liver graft, "reperfusion" and additional work on the graft, and then closure. For a variety of studies and discussions, we break the operation down into three stages. Breaking the procedure into these stages will permit a better delineation of specific anesthetic goals because of the changing physiology.
The pictures below show the difference in anastomoses between a conventional bicaval technique versus a piggyback technique.
[Nature Reviews Gastroenterology & Hepatology 10, 434-440 (July 2013)]
Stage I: "DISSECTION Phase"
This will consist of incision, exposure, dissection, and recipient hepatectomy. In another context, consider it major abdominal surgery in the presence of coagulopathy, portal hypertension, and frequently severe scar tissue from prior hepatobiliary surgery or from post-necrotic hepatitis. The goal of this portion of the surgery is to mobilize the vascular structures around the liver (suprahepatic vena cava, infrahepatic vena cava, portal vein, and hepatic artery) and isolate the common bile duct. In addition, adhesions between the liver, diaphragm, and retroperitoneal areas must be dissected to allow full mobilization of the liver within the right upper quadrant. Be wary of the effects of hepatic manipulation on venous return: i.e., surgically induced hypotension. This must be distinguished from other causes of hypotension, which are all too common during liver transplantation.
Toward the end of this stage, we prepare for the anhepatic period by a technique of volume loading if a bicaval clamp is planned --- administration of colloid solution and/or blood (titrated to filling pressure, e.g. CVP of 10- 15 mmHg)in anticipation of a significant decrease in filling pressures after the caval clamps are applied. However, until just before cross clamp, low CVP is preferred (≈ 5 mmHg if tolerated) to reduce blood loss. In addition, a general goal during the dissection phase of the operation is to promote a diuresis. Diuresis is usually necessary to allow transfusion of sufficient FFP without creating excessive volume overload and hemodilution.
Stage II: "ANHEPATIC Phase"
This is the period of time when no liver is in the circulation. At UCSF, this is nearly always managed without the use of venovenous bypass, and consequently, this stage has significant metabolic and cardiovascular implications. This is the most challenging part of the anesthetic management.
The hemodynamic considerations depend on the planned surgical procedure. The bicaval technique is more challenging from a hemodynamic standpoint and will be described below:
The stage begins with the applications of multiple vascular clamps: 1) Hepatic artery 2) Portal vein 3) Infrahepatic Vena Cava 4) Suprahepatic Vena Cava. The mobilized liver is quickly removed, and the ice-cold liver graft is placed in the field. During the next 30-45 minutes, 3 anastomoses are completed: 1) Suprahepatic vena cava (right beneath the diaphragm, look over and see the hepatic veins draining into the cava of the new liver), 2) Infrahepatic vena cava, and 3) Portal vein --- a smaller vessel but critical for providing flow into the liver. Fluid management with the caval clamps on is extremely difficult, and we must anticipate the return of volume when the clamps are released at the completion of the vascular anastomoses. This must be balanced with use of pressors and inotropes. Currently, a staged- -release is performed: the suprahepatic caval clamp is released and the anastomosis is inspected and small leaks repaired. The infrahepatic caval clamp is then released and the anastomosis is inspected and repaired if needed. The filling pressures will normally reflect the establishment of caval continuity. Finally, the portal vein clamp is removed allowing recipient blood flow into the donor liver. At this point, the anhepatic period ends and the next phase begins.
Stage III: "REPERFUSION Phase"
This covers the time from vascular reperfusion of the liver graft until the end of the procedure. The actual moment of reperfusion can have profound cardiovascular effects (i.e. arrhythmias, profound hypotension, and about a 3-5%incidence of hyperkalemic or other cardiac arrest) and anesthetic management is directed to maintaining or recovering cardiovascular stability. Multiple factors probably combine to produce this hemodynamic insult: ionic composition of the preservative solution, hypothermia, metabolic acidosis, "vasoactive" peptides from the gut, other cytokines, sudden atrial stretching in response to the unclamping and reperfusion. Initially, profound bradycardia usually occurs, which can become asystole. This can be treated by glycopyrrolate or atropine, but we usually use epinephrine in small doses, 10-30 μg at a time. After the initial bradycardia, the hypotension appears to be overwhelming vasodilatation (very low SVR) with adequate filling pressures and high cardiac output (15-20 L/min).
Following reperfusion, attention is paid to the diagnosis and management of a significant coagulopathy (dilution/consumption of clotting factors, platelet entrapment, heparin effect, primary fibrinolysis), and resultant bleeding. Platelets should be administered to correct thrombocytopenia. Most of the necessary treatment is FFP. Antifibrinolytics can be given if fibrinolysis is suspected. Protamine can be administered to treat any heparin effect. Antifibrinolytics, prothrombin complex concentrate, fibrinogen concentrate, factor VIIa, and protamine should be discussed with your attending and the surgeons prior to use. Laboratory analysis and blood product support guide the management of the coagulopathy. Bleeding tends to be of mixed, multifactorial etiology: coagulopathy as mentioned above, extensive raw surfaces above and behind the liver, effects of portal hypertension, and multiple anastomoses.
In addition, we monitor evolution and evaluation of liver graft function (base deficit), and recovery from renal ischemia. During this time the surgeons are completing the hepatic artery anastomosis, controlling bleeding, performing a cholecystectomy on the liver graft, and providing for biliary drainage via an end-to-end (duct-to-duct)anastomosis or a choledochojejunostomy with a Roux-en-Y loop.
Toward the end of the procedure, we shift the anesthetic emphasis to consider postoperative disposition. One option is to provide analgesia and sedation in anticipation of postoperative mechanical ventilation. If the liver is functioning adequately, and the patient is hemodynamically stable with adequate gas exchange, tracheal extubation should be attempted. This has been very successful in a high percentage of patients. Hypertension may develop toward the end of the procedure in about 20-30% of cases. Relative hypervolemia, activation of endogenous reflexes during the anhepatic period, and possibly activation of the renin/angiotensin and endothelin systems probably combine to produce the relative hypertension. It is important to identify a tendency to hypertension before leaving the operating room and provide treatment since hypertension could promote bleeding at the arterial anastomosis.
Intraoperative Hemodynamics: A General Depiction
During the liver transplant procedure, a general pattern of hemodynamic changes usually evolve in each patient. This typically reflects the common cardiovascular changes that accompany chronic liver disease, and the manifestations of "reflex responses" to the anhepatic period, and the acute effects of reperfusion as described above. Below, data gathered in one patient during the critical part of the operation are displayed: end of dissection phase, anhepatic period, and reperfusion.
Please understand that this is the data from one patient, a 60-year-old woman with primary biliary cirrhosis, with the usual manifestations of end stage liver disease. These values are representative for a typical patient undergoing liver transplantation. Note that the initial cardiac output/index is evidence of a hyperdynamic circulation associated with a decreased systemic vascular resistance. During the anhepatic period, marked reductions in filling pressures and cardiac index do not result in systemic arterial hypotension. The ability to increase the SVR three- or four-fold results in a relatively stable blood pressure. Not all patients will demonstrate the same reflex responses to the anhepatic period, and support of blood pressure may require additional volume and/or pressor support. Not evident in this data are the acute and transient changes that occur upon reperfusion of the liver graft. Despite a sudden increase in central filling pressures, a period of hypotension (2-3 minutes) usually accompanies graft reperfusion, and usually requires immediate intervention (consider: prophylactic calcium, blood pressure support with phenylephrine, norepinephrine, or epinephrine.)
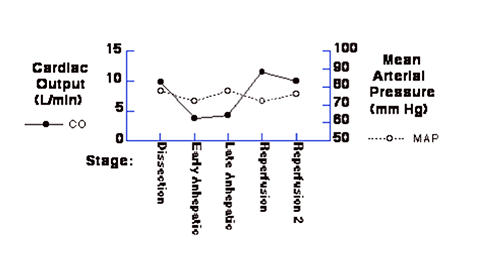
|
Stage |
HR | MAP | CVP | PA | CO | CI | SVRI |
|
Dissection (II-5) |
84 | 78 | 5 | 19 | 9.9 | 5.8 | 589 |
|
Anhepatic (II+10) |
99 | 72 | 2 | 4 | 3.8 | 2.2 | 1472 |
|
Anhepatic (III-5) |
96 | 78 | 5 | 5 | 4.3 | 2.5 | 1356 |
|
Reperfusion (III+10) |
86 | 72 | 7 | 21 | 11.5 | 6.7 | 452 |
|
Reperfusion (III+30) |
91 | 76 | 8 | 22 | 10.0 | 5.8 | 543 |
Anesthesia for Liver Transplantation: The Basic Game Plan
Unlike elective operations, we can never say who we will be transplanting or when the case will start. As mentioned earlier, the cases will be scheduled when a donor liver has been identified. Because of multiple logistic considerations regarding the procurement of the donor liver, do not be surprised if the scheduled "Start" time is changed.
It is important to discuss the various risks with the patient, and specific mention should be made of transfusion and post-operative intubation. The anesthesia attending should complete this discussion with the patient.
As with any operation, there is no ideal anesthetic ---there have been no controlled trials comparing different anesthetic agents or techniques during liver transplantation. Anesthetic induction can be performed with Propofol. Desflurane, isoflurane, or Sevoflurane for maintenance is adequate, with opioid (usually fentanyl). Any reasonable anesthetic technique is possible, but must take into consideration the intricacies of intercurrent illness (i.e. hepatic failure to some extent) and surgical requirements (in this case management of the anhepatic phase without the use of venovenous bypass). Central to our anesthetic management is preparation and anticipation: at critical points of the operation we look for and expect certain responses from the patient, and are ready to intervene during those critical moments, mostly during the anhepatic phase and with graft reperfusion.
An arterial line is absolutely essentially. This is usually placed in the left radial artery, because this arm can remain out, while the right arm is tucked. This leads to many fewer problems. Femoral arterial lines can be necessary. A right IJ 7 or 9 Fr introducer should be placed, with a PA catheter if indicated, or alternatively with a DLIC. A left antecubital RIC (7.0 Fr) is the preferred volume line, which can be used by the rapid infusion device (Belmont).
Laboratory and Blood Sample Protocol
There is no shortage of intraoperative laboratory tests during this operation. In general, arterial blood samples are sent at baseline and serially during the operation. During critical phases of the operation, labs are usually sent every 30 minutes. We now have the ability to do point of care ABG analysis, which has eliminated problems with delays. This also provides complete electrolytes, glucose and lactate. CBC and coags are sent to the main laboratory.
Fluid Management
It is fairly common to expect a blood loss of 1-1.5 blood volumes (i.e. 5-8 liters), and the total intravenous fluids administered may be 15-20 liters. This fluid will consist of crystalloid, colloids, blood products, and "rapid infusion" support. The rapid transfusion device is an important part of the anesthetic management and support of the liver transplant patient. At UCSF, we have a technician available for all of our cases, and following the induction of anesthesia, they can prepare up to 1-2 liters of washed packed red blood cells (washed to remove K+ and acid) or modified whole blood consisting of washed packed red blood cells reconstituted with fresh frozen plasma. This solution is administered via a Belmont, usually through a left antecubital 7.0 Fr RIC. We are usually able to obtain flow rates of 300- -600 cc/minute, and the perfusionist will administer this volume under the direction of the anesthesia team.
Standard blood products are set up for liver transplant. This includes 5 or 10 u PRBC and 5 or 10 u FFP to be brought to the OR, with 2 or 4 single donor platelet units available on request. If there are any questions about blood banking issues from the circulating nurse, please refer them to your attending. Blood products are brought to the OR in coolers and the platelets in a soft blue insulating package. The nurses and perfusionist will check these in for us.
The Liver Anesthesia Team (as of September 2022):
Kate Kronish, MD (Division Chief)
David Shimabukuro, MDCM
Dieter Adelmann, MD, PhD
Helge Eilers, MD
Hung Nguyen, MD (pediatric)
Irfan Kathiriya, MD, PhD (pediatric)
John Feiner, MD
Linda Liu, MD
Manny Pardo, MD
Michael Bokoch, MD, PhD
Nicholas Mendez, MD
Steve Weston, MD (adult/pediatric)
Acknowledgements
Significant contributions have been made by current and former members of the liver transplant service: Helge Eilers, John Feiner, George Gregory, Sonali Joshi, Linda Liu, Claus Niemann, Manny Pardo, David Shimabukuro, Stephen Weston, and Spencer Yost.
This information is meant to serve as an educational resource. Clinicians should use their own professional judgment in the care of any individual patient as the guidance contained in this document may not be appropriate for all patients or all situations.